Cuba tests vaccine candidate Abdala against COVID-19
TeleSur | Saturday, 5 December 2020 | Click here for original article
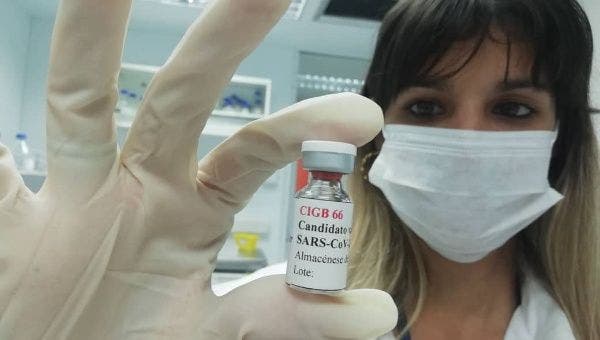
The clinical trial of the vaccine candidate Abdala began on December 2 in Santiago de Cuba | Photo: Twitter / @CIGBCuba
So far, Cuba has four vaccine candidates: Soberana 01, Soberana 02, Abadala and Mambisa.
Cuba has begun the clinical trial of a new vaccine candidate against COVID-19, named Abdala (CIGB-66), in Santiago de Cuba province, in the east of the country.
The candidate was developed by the Center for Genetic Engineering and Biotechnology (CIGB) of Cuba, an institution that is dedicated to the research, development, production and commercialization of products of this branch.
On Friday, the CIGB announced the start of the trial, specifying that the process is in "the first phase of the clinical study" and will run until February 16, 2021.
This phase of the trial "has 200 volunteers and involves more than 40 health professionals, including doctors, nurses and laboratory workers," indicated the CIGB.
For her part, the head of the Department of Teaching and Research of the scientific institution, Liudmila Risset Castro, told local media that the process includes the intramuscular administration of the doses starting next week.
The action will be centered at the Saturnino Lora Hospital, in Santiago de Cuba, with the aim of testing the effectiveness, efficacy and immunogenicity of Abdala (CIGB-66). As part of the preparatory process, all adult subjects, healthy or with controlled chronic diseases, were rigorously evaluated in advance, Risset Castro said.
Abdala is the result of the work of researchers from the CIGB, in the Cuban capital. The candidate recently received authorization for this phase from the Center for State Control of Medicines, Equipment and Medical Devices (Cecmed).
Trials for Soberana 01 and 02 are already underway and results are expected in early 2021.






